Automated report compilation
for truly searchable PDF reports
Merge documents into a single
compliant PDF from your DMS & RIM platform.
See How DocShifter’s Reports+ Works - In Just 4 Minutes
Benefit from automated report generation and report compilation
that saves you time and money
Spend more time on content rather than on form
Reduce the number of manual errors caused by decentralized report creation
Deliver quality-assured, (compliant) reports faster and save time in content preparation
Remove the reliance on complex publishing software for simple report creation
Uniform, high quality (compliant) PDF reports
Improve operational efficiency with trusted automation
Designed to make your
report publishing fully automated
- Authors store their documents as usual. A DocShifter workflow will pick up the document automatically and convert to the right format, at the right time
- Workflows automatically route content based on the type of file, metadata, template used, signed or not signed documents, and much more
- A simple to use and highly flexible workflows to define your automation steps
- Check for new documents from multiple content sources (see DS Connectors to find out more)
- Merge multiple documents in different file formats, including ZIP files, OpenText’s Documentum Virtual Documents, and Veeva Binders
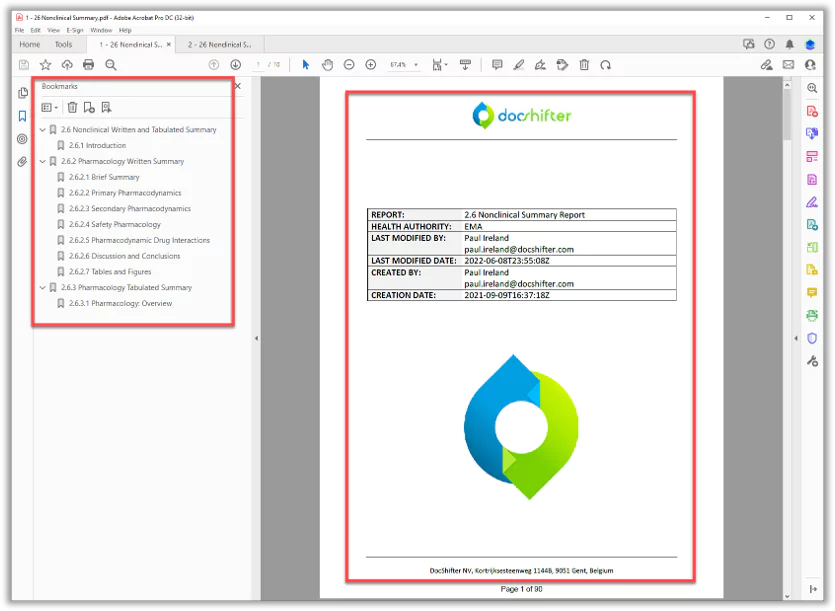
- Automatically add cover pages to your reports
- Add cover pages at the start and end of reports
- Add cover pages at the start or end of each report volume (where output is more than one PDF file)
- Template designs directly in Microsoft Word
- Generate tables of contents for entire reports
- Generate tables of figures for entire reports
- Handle all content, table and appendices tables
- Use attributes and metadata from your document management system
- Include dynamic titles and visuals in your cover pages, tables of contents, headers & footers, and more
- Add consistent pagination including page and volume numbers, and totals
Want to see automated report publishing in action?
Contact us.
Do you recognize these challenges?

Manually merging documents costs both time and money
Creating a single report from multiple source documents is a tedious task. Adding a cover page, adding a ToC. All of these take time and a lot of manual steps. Automated report generation solves the challenge, saving businesses both time and money.

Standardizing multiple documents into a single PDF report is risky
Cover pages with logos, graphics, texts, table ofcontents, figures and even tables of tables – standardizing disparate documents into a seamless PDF report is often anything but easy. Being able to automatically merge documents into PDF reports ensures quality and reduces human error.

Science takes time. Your reporting shouldn’t.
Creating single reports from multiple individual source documents can be unnecessarily complex and expensive to manage. Whether it’s for clinical-study reports or general document-level publishing, finding the right solution to automate and generate regulated reports is not just a nice to have, it’s business critical.
The solution? Automate and centralize your report level publishing and report compilation
DocShifter’s Reports+ solution generates seamless PDF reports by merging multiple documents into one or more (compliant) PDF reports ready for regulatory publishing.
By automating the manual steps typically taken to create or compile a report – adding a cover page, tables, watermarks, pagination, headers & footers, and just about everything else you need – Reports+ automated report generation reduces the time and financial burdens associated with manually collating and finalizing reports.
The result? Confidently merged and consistently (compliant) PDF reports.












Automated Report Level Publishing for 510k and PMA Submissions for a Medical Device Company

“DocShifter takes all our ZIP files and various documents from our document management system; and creates a large PDF for our device submissions.
The final PDF includes all key elements like page numbers, bookmarks, a cover page, table of contents, headers & footers.
Everything happens automatically once we define the content for the PDF report.”

Speak to one of our specialists
Frequently Asked Questions
How are DocShifter’s automated report generation capabilities complementary with LORENZ Docubridge’s publishing capabilities?
DocShifter’s report generation functionalities are fully automated features. For example, they enable the fast creation of PDFs that would typically be included in a submission using LORENZ’s publishing tools.
Can Reports+ handle regulatory submissions such as 510k and PMA for medical devices?
Yes. DocShifter's automation capabilities allow the creation of 510k and PMA submissions by merging multiple documents into a single, compliant PDF file.
Can DocShifter automatically bookmark PDFs and create Table of Contents (ToC)?
Yes. PDFs are fully bookmarked, with Tables of Content created automatically as necessary (as well as cover pages, pagination, and more)
Based on what can DocShifter split PDF reports automatically?
DocShifter can automatically split reports based on either file size, or page numbers.
Can I use the metadata in my document management system to populate information in the final PDF report?
Absolutely. DocShifter can utilize the metadata in your DMS to populate information in the final PDF report.
Can we trigger automation in our DMS / RIM with DocShifter when individual documents reach a certain lifecycle?
Yes. The idea is that DocShifter handles all the document merging / report compilation steps, in a fully automated way.
Please contact us about which document / regulatory information management system you use, and we will be happy to discuss automation trigger options with you.
When combining submission docs into one submission ready PDF, can you combine various doc types, such as word, excel and pdf docs?
Yes. DocShifter can can take multiple file formats, convert these to PDF and create 1 PDF report including all source files.
The final PDF report can also be submission-ready with the right bookmarks, hyperlinks, table of contents, page numbers, fast web view, viewing options and so on.
How about ZIP files?
Absolutely. You can, for example, use DocShifter Express' user-friendly UI to drop a ZIP file and receive a merged PDF document back in seconds.
You can check this short video out to see some capabilities around handling ZIP Files: https://youtu.be/o5uysDVThZ4?si=D7YfF6BMPo8Sb6fw&t=1670