Here are 5 ways to automate submission content preparation in Veeva with DocShifter
1. Achieve document compliance earlier in your submission process when using DocShifter with Veeva (or any other repository)
A typical drug development process takes from 5 and 15 years, and documentation is created every step of the way. Marketing Authorisation submissions are compiled at the end of this timeframe. Documents are typically only checked for compliance either just prior to or after a draft submission is published. What if your original source document needs to be updated?
Do you really want to be updating source documents, or editing your individual PDFs to ensure each and every document complies with the technical specifications laid out by the Health Authorities? When you are trying to focus on getting the submission out the door! Many of the source documents can be hard to even find.
What we mean by achieving compliance earlier, is to ensure that any issues are identified much earlier in each document’s lifecycle: at the time the source file is saved; or at the time the PDF version is created. It means using a solution to generate fully technically compliant PDFs as soon as the source document is available, and not relying on other tools and plugins to achieve that level of compliance. Any remaining issues that require an update on the original source document can be identified automatically at source or as the PDF is generated. Rather than manually checking each PDF is submission ready, that check-list can be automatically created during the process.
The message we try to get across (and where our customers see the biggest difference) is that by achieving document compliance earlier in this way, you can reduce the risk of missing submission deadlines and of issues from having manual steps in your process. Problems can be addressed weeks, months, or even years earlier in the development process and the entire submission creation process simplified.
How does this impact your operations?
- No last-minute rushing to ensure PDF compliance. The PDFs you create will already be compliant.
- Accelerate preparing your regulatory submissions by removing manual steps.
- Identify any remaining issues earlier.
- Focus on the content, not ensuring technical compliance.
- Reduce risk of non-compliance and missed deadlines.
2. Let authors focus on the content, and let DocShifter take care of the formatting & styling
Today, authors are not only tasked with adding content to their Word documents, but are also tasked with making sure that the formatting and styling of Word documents are accurate. Even with proper templates in place, this is a process that does not always go as planned.
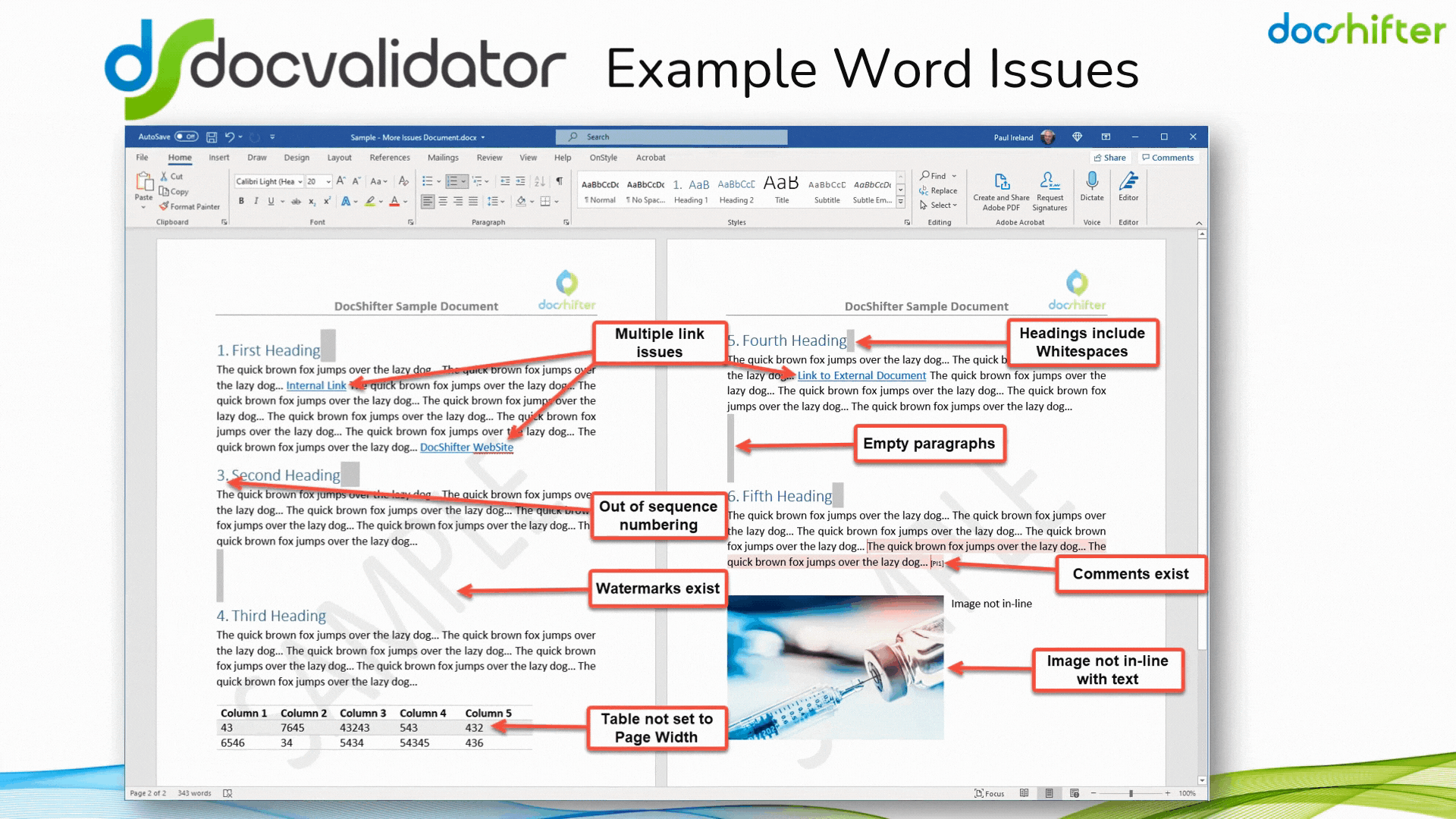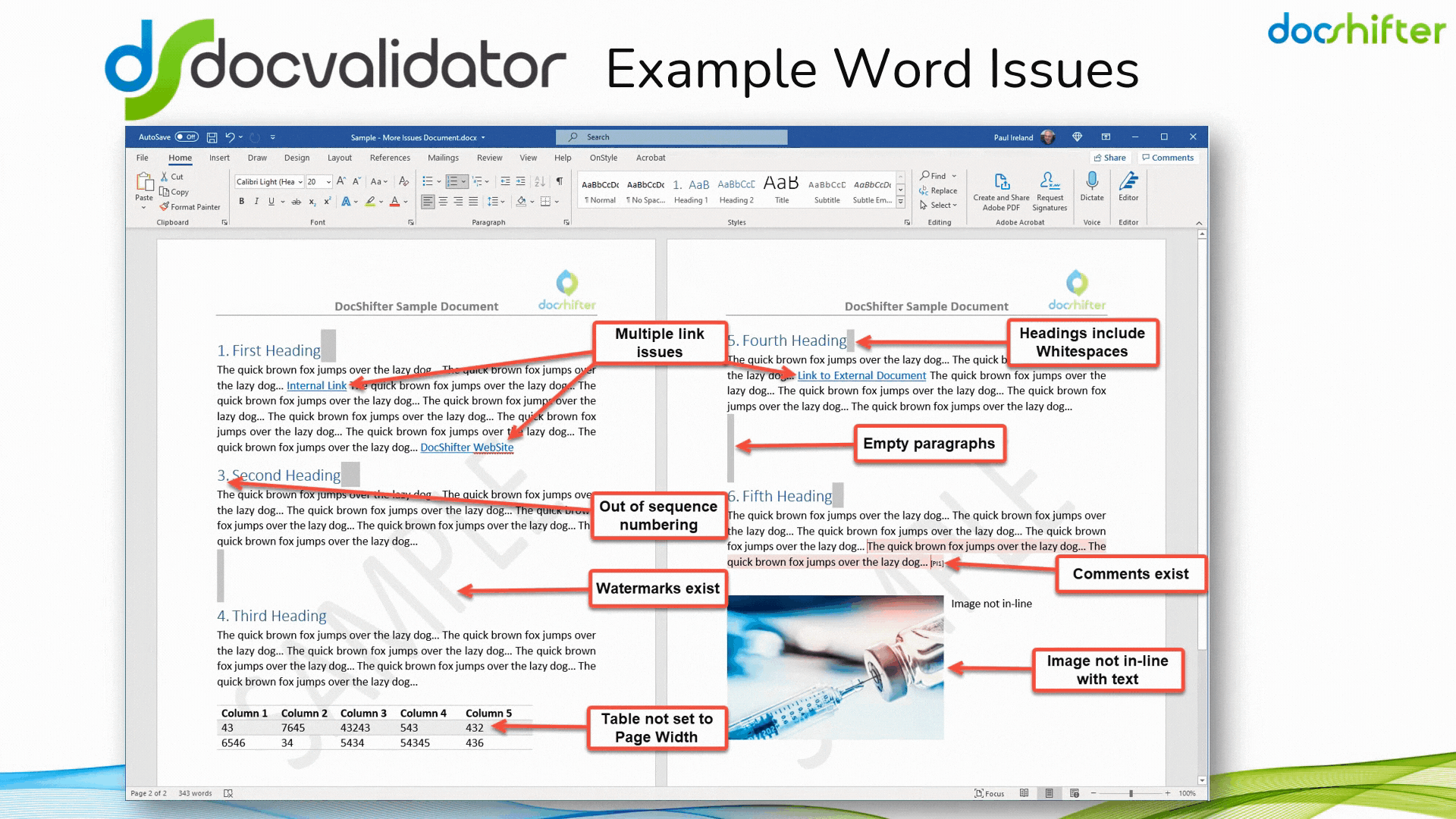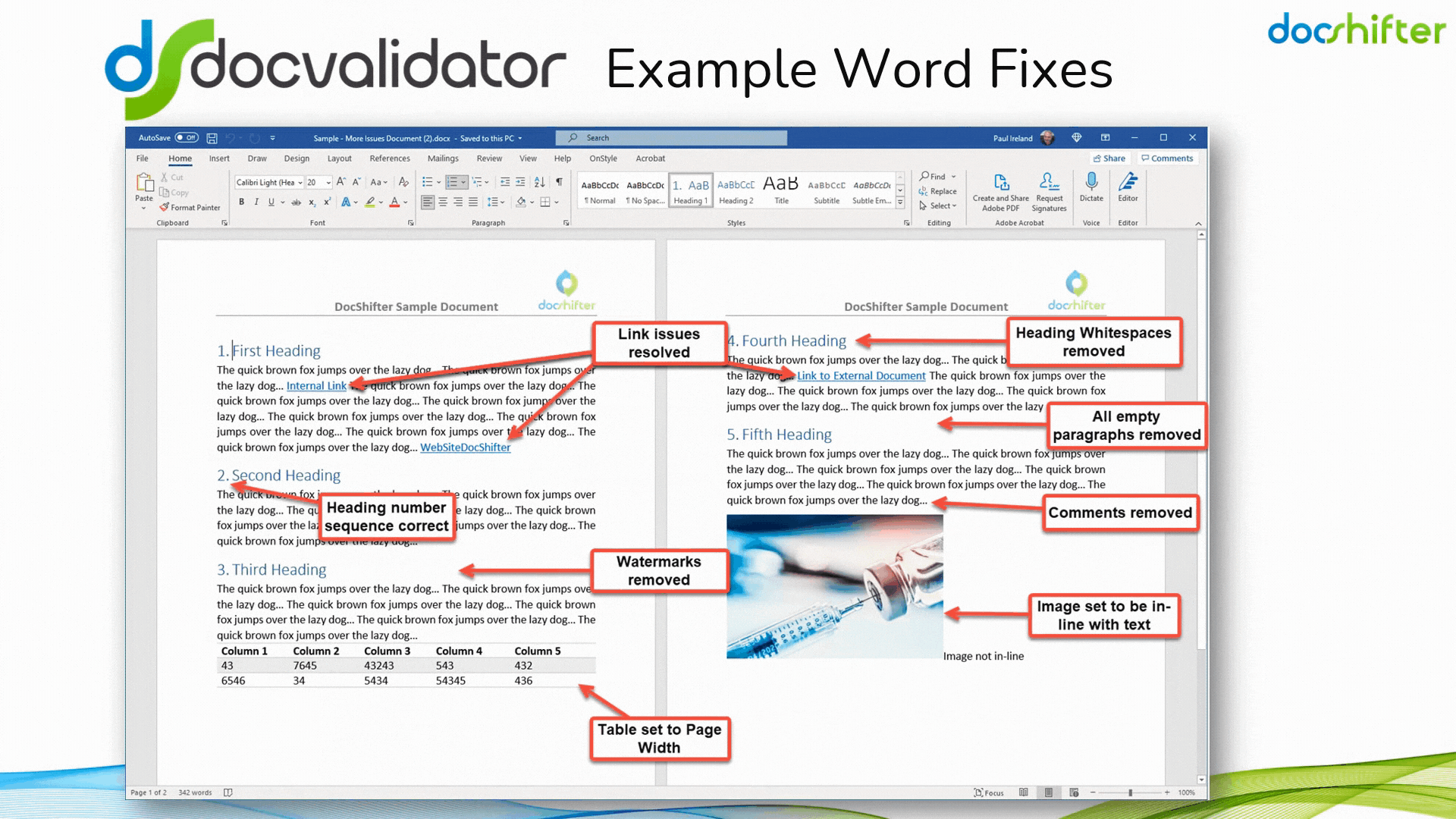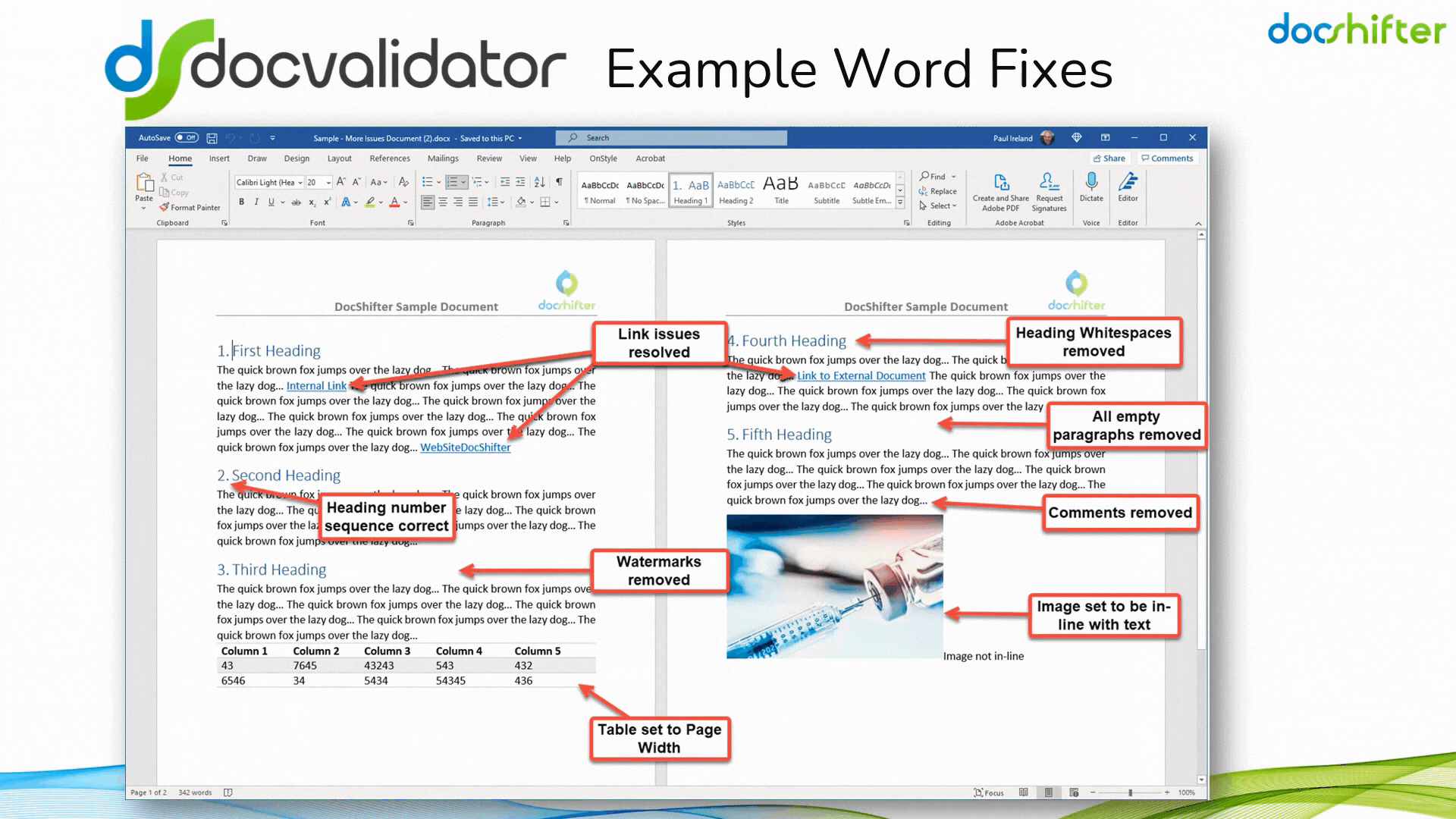
Did you know that DocShifter’s DocValidator can automatically identify, report & fix issues with many formatting and other errors in your Microsoft Word documents? The software can automatically find, report and fix issues with links, headings, paragraphs, tables, images, styles, comments, track changes, etc. The report, or the fixed document, is then automatically stored in Veeva and individuals alerted of the results.
With DocShifter’s DocValidator in place, your authors can focus on what matters most; the content.
3. DocShifter utilizes metadata in Veeva to create multiple global PDF renditions
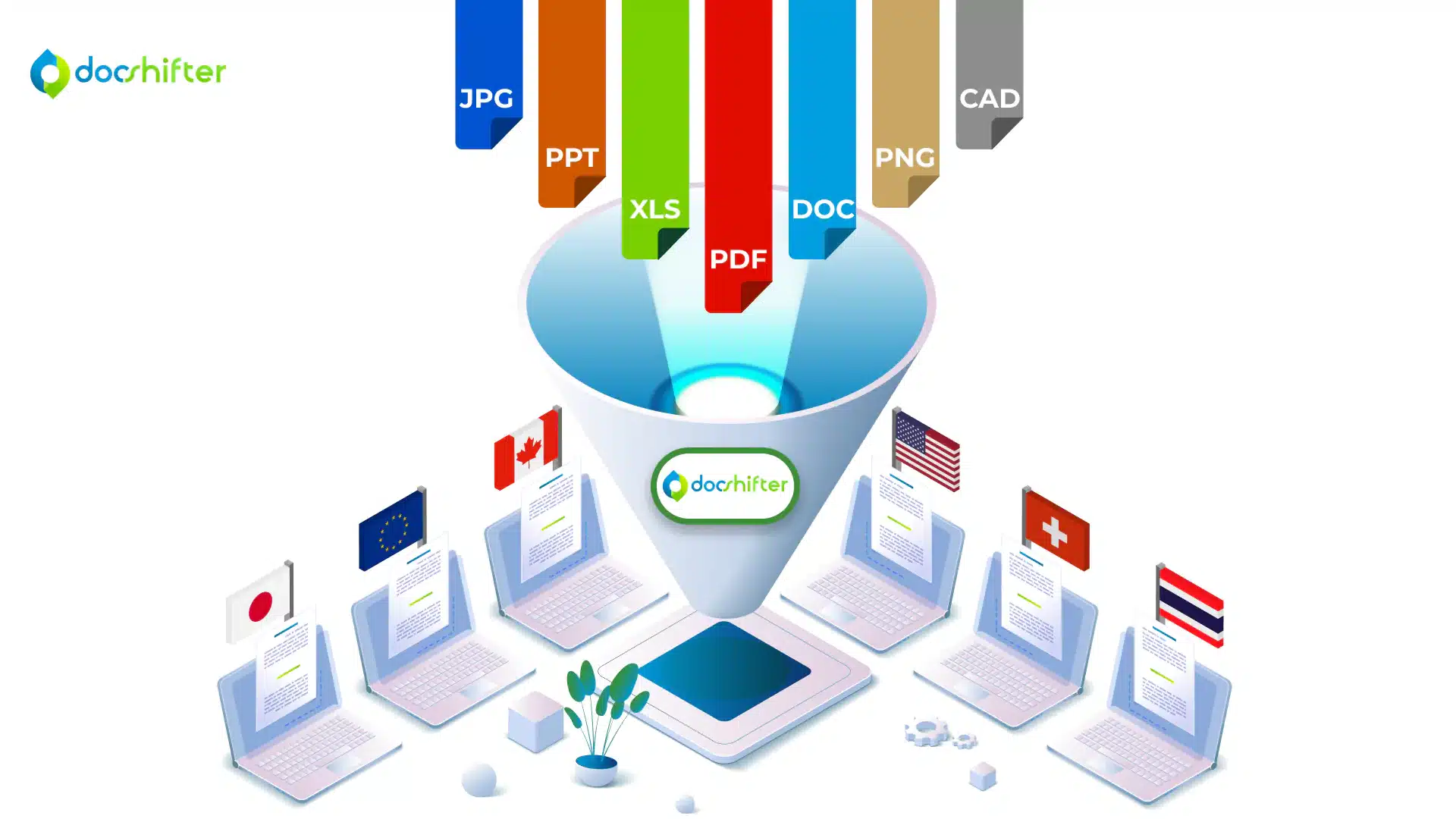
From conversations with RegOps teams from all over the world, we understand that creating compliant PDF renditions for a health authority is a challenging task. Most of the time, once a PDF is created, teams rely on other tools and plugins to achieve compliance. Multiplying all that work for multiple health authorities, and creating multiple compliant PDF renditions becomes an even bigger nightmare.
With DocShifter, you’re able to generate simultaneous global renditions. The software can automatically create one single rendition if you choose to just have one common rendition globally. But it can also create multiple simultaneous renditions; each one meeting the specification of a different health authority: FDA, EMA, PMDA, Swissmedic, HC, etc.
As an example, you can have one source document and DocShifter will create a separate PDF rendition for each health authority where it is required. To achieve this, DocShifter leverages any available metadata you have available in Veeva. DocShifter can check the value of the metadata to decide if a rendition is required for each region or country you market or plan to market your products in.
This automates many steps in your process that you might currently be performing manually.
4. Compile documents in Veeva to automate your report generation process
During our latest webinar, we also touched upon generating reports from documents, ZIP files or Veeva binders. You can set up DocShifter to monitor the status of binders and ZIP files to merge multiple files together that are stored in Veeva.
A report can contain various elements: cover pages, a table of contents or table of tables/figures, headers & footers, pagination, watermarks, etc. DocShifter can fully automate all these things using existing Veeva metadata.
Is it an internal report, such as an SOP, Annual Report or a training guide? Or is it a clinical study report you are looking to include in your dossier? If you are, the document needs to comply with any health authority requirements like any other PDF.
As well as automating the generation of your report, DocShifter can also split the results based on the number of pages, or file size, depending on where you are submitting the content. This ensures you meet specific regulatory requirements faster, with less human interaction involved, and with less risk of error.
5. Let DocShifter show you the non-compliant elements in your PDF content in Veeva; and fix issues automatically
“The problem is not actually rendering. We create those PDFs anyhow. The problem is us spending hours and hours of time on editing PDFs with tools like ISIToolbox. Especially not knowing what was wrong with the PDFs. And that is what DocShifter is now doing for us.”
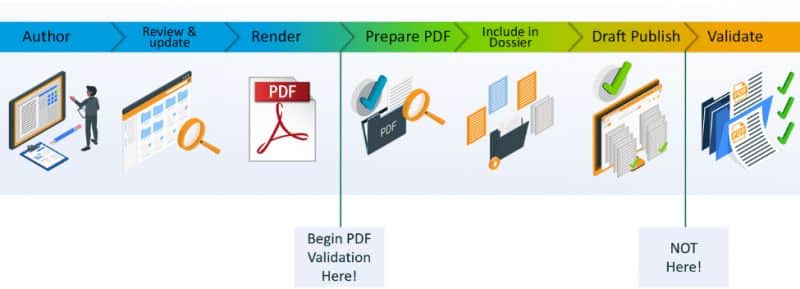
Do you recognize this as well? . During our webinar, we talked about how automated PDF validation can accelerate your submission preparation time.
DocShifter’s PDFValidator can check and analyze any PDF content, regardless of how and where it was originally created. The software looks for issues with hyperlinks, bookmarks, PDF version, security, optimization, font embedding, etc. in your PDF files.
Rather than using multiple tools and plugins to manually identify these issues, PDFValidator automatically checks your PDFs and stores the results as a report back in Veeva. Many of the errors can also be automatically fixed and a new PDF version created. The report will identify any remaining issues so you can make any final changes a lot faster: saving you valuable time. How many errors were there in the PDF? What type of errors were there? Where are the errors in the PDF?

How to automate document preparation & report generation in Veeva with DocShifter