- Following our previous “What’s next after eCTD?” article, this paper aims to provide a brief update on the next major version of eCTD, version 4.0. Since 2003, eCTD has been adopted by an increasing number of Health Authorities around the world. The standard has evolved incrementally in that time. eCTD 4.0 will introduce significant updates that address many of the limitations and frustrations that both agencies and sponsors alike have identified over the last 19 years.
Who is (unfortunately) old enough to remember this?
Date: March 2007.
A submission. 500 binders for Module 5, 60 binders for Module 4, 30 binders for Module 3, 10 binders for Module 2, and 2 or 3 binders for Module 1.
In total 600 binders, each 300-350 pages totaling about 200,000 pages. Each box contains 5 binders, a pallet contains 18 boxes, so my guess is you are looking at 3 (perhaps 4) copies.
Thank you electronic submissions for taking this burden away. And thank you eCTD 4.0 for further improving the standards.
Summary
eCTD 4.0 is based on the Health Level Seven (HL7) standard called RPS (Regulated Product Submission). This standard focuses on simplifying the processing and review of regulated product information rather than the actual content itself, which will continue to develop with each iteration of the eCTD. RPS was also designed to be used in other sectors to gain approval for medical devices, animal health and other regulated products in the future.
The primary goals of eCTD 4.0 are to:
Implement changes that speed up the regulatory submission process.
Enhance how agencies and sponsors communicate.
Improve global harmonization of the format.
eCTD 4.0 Timelines
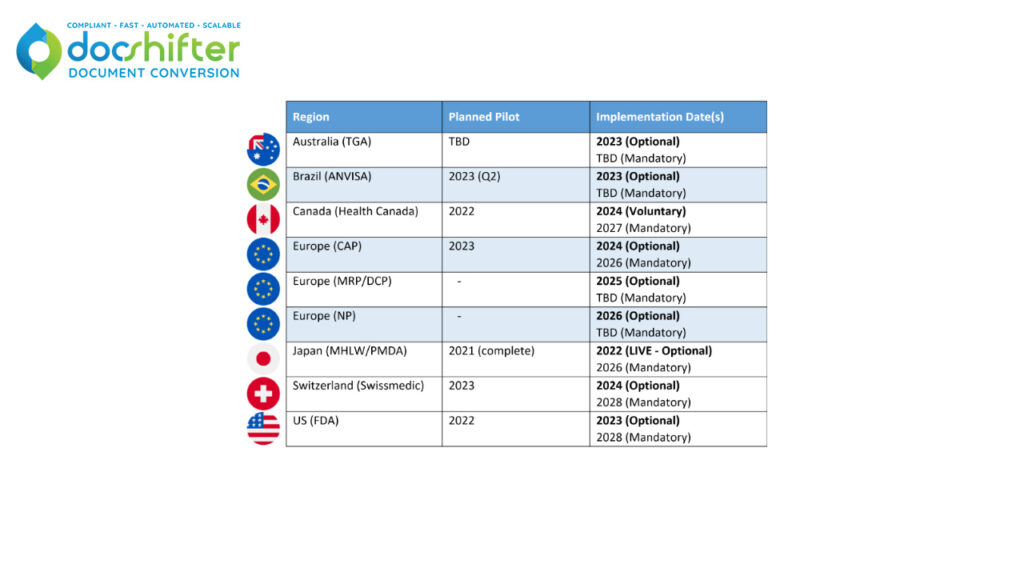
Despite numerous delays due to the global pandemic and other hurdles (the FDA for example planned to begin their initial pilot in 2015), a number of health authorities have completed, started or planned pilots for their implementation of eCTD 4.0.
*Please note that this image was created on 28/09/2022, and is subject to change.
The ICH published the implementation guide for eCTD 4.0 in 2018 with minor updates in June of last year. Japan- who completed their pilot last year- will be the first to begin accepting voluntary applications in the new version this year. Brazil will begin accepting applications for the very first time in eCTD directly to the version 4.0 specification from next year. By the end of 2023, currently Australia, Brazil, Canada, Japan and the US will all be accepting applications in the new version, with the EU and Switzerland starting the following year.
With the US planning to begin accepting eCTD 4.0 submissions sometime in 2023 (if the testing phase goes well) the LORENZ validation tool in use at the FDA is expected to be ready by March 2022. Initially, the use of eCTD 4.0 will not be mandatory in any region and typically only be an option for new applications. An overlap period is expected when both eCTD 4.0 and 3.2.2 submissions will run in parallel, with each country defining their own grace period of between 2 – 5 years before mandating the new version’s use.
In addition to the delays, not all aspects of eCTD 4.0 are expected to be implemented from day one. The two-way communication, which is seen as a key component of the new version, will not be implemented initially in the US, for example. Future phases will incorporate this capability and processes to continue applications initially submitted in the 3.2.2 spec in the 4.0 specification. These will be critical to the long-term success of the format.
Key Changes in eCTD 4.0
While there will be some process changes required by the sponsors, most of the burden will be placed on the publishing and reviewer vendors to adapt their solutions to the new standards. There will be more reliance on technology by all parties to interpret the information being provided; it will no longer be possible to use a web browser to open your submissions, for example. As with any significant change to the submission standards, there will likely be a number of sponsor organisations looking at their current solution providers to ensure they still have the best technology for their needs.
To achieve the primary goals of the new specification, many updates will be implemented.
Implement changes that speed up the regulatory submission process.
Document re-use.
It is common for the same content to be needed multiple times throughout the lifecycle of an application. Currently, this involves submitting the same document in each sequence that it is required.
With eCTD 4.0 each document will be assigned a Universal Unique Identifier (UUID). This identifier can be referenced in future sequences, avoiding the need to resubmit the content itself.
Lifecycle enhancements.
To improve the management of content over longer periods of time, it will be possible to replace many documents in the application with one, or to replace one document with many. The legacy ‘append’ operation will be officially eliminated in eCTD 4.0, and it will be possible to rename documents and metadata (e.g. update the trade name).
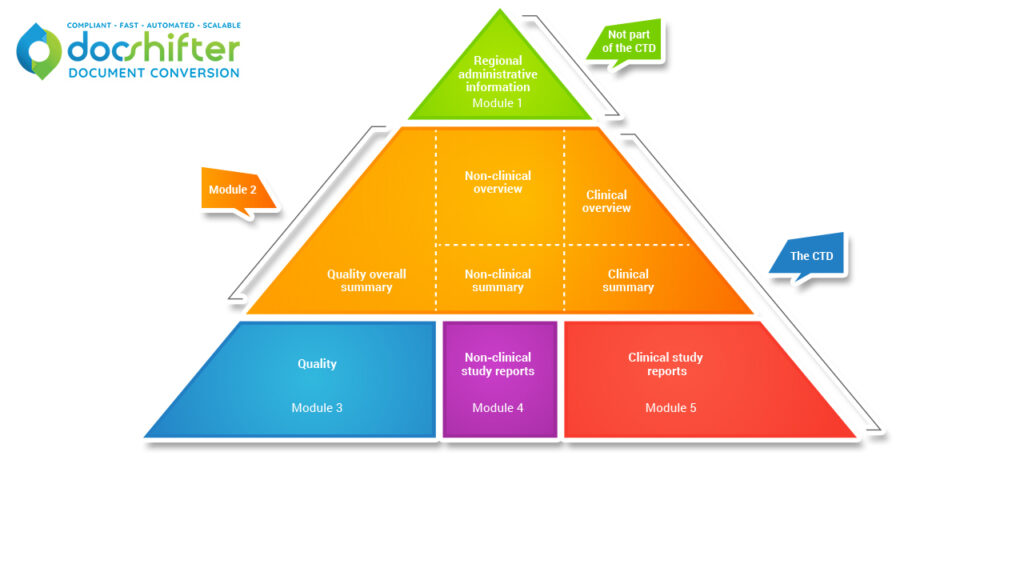
Tables of Content.
The hierarchy set out in eCTD v3.2.2 will become a flat structure in eCTD 4.0. Context of Use and Keywords will be used instead to define the content’s location within any eCTD tables of content generated by the viewing tools.
Study Tagging Files (STFs).
Document Groups will replace the need to use Study Tagging Files, which will no longer be used in eCTD 4.0.
Enhance how agencies and sponsors communicate.
Two-way communication between sponsor and agency.
The current specification of the eCTD only allows for one-way communication from the sponsor to the agency via individual sequences. All communication from the agency to the sponsor is done separately and does not form a part of the eCTD itself.
eCTD 4.0 will also enable the agency to respond to the sponsor via a sequence, creating a full picture of the entire lifecycle of the application, including any questions or requests for information, in one single place.
The use of controlled vocabularies.
The increased use of agreed controlled lists is seen as an essential step in ensuring consistent communication between sponsors and agencies. These lists will be controlled by various bodies, including regional authorities, ICH and other third parties. The publishing systems will need to implement these.
Improve global harmonization of the format.
Standardization of the format with multiple Standards Organizations.
As well as the ICH, eCTD was also developed based on the HL7’s RPS (Regulated Product Submissions) project, with an aim to become an ISO standard. Along with the involvement of Health Authorities and other third parties for controlled vocabularies, the long-term vision of sponsors and agencies is to provide more content from eCTD dossier via structured data (such as that set out in the IDMP standard). This should all lead to improved harmonization of how the eCTD is implemented across different health authorities, where currently there are more differences than envisaged.
A new XML schema.
This has been designed to support the updated features of the new standard and be more flexible in the long-term.
Impact on Submission Content Preparation
The PDF format will still be required for much of the content provided in the eCTD. Some minor changes to the ICH guidelines for PDF use have been defined, so it is important to ensure that the tools used for PDF generation can continue to provide compliant results. The compression method suggested for images has been adjusted for example, so sponsors should ensure they can produce PDFs to the correct new PDF specifications.
Video Recording: eCTD 4.0: What is Changing?
On September 29th we discussed eCTD 4.0 during a LinkedIn live session. We touched on topics such as the revised eCTD 4.0 implementation timeline and what changes it brings.
What can you expect in the video?
- What is eCTD 4.0?
- How is it different from previous versions?
- By when do we need to be ready?
- Why is it important?
- What are the benefits for the sponsor organizations?
- How does it affect the content required for a submission?
- Are there any drawbacks?
Conclusions
As eCTD 4.0 becomes a reality, the industry as a whole will finally begin to benefit from some much-needed changes to the regulatory submission process. This in turn should lead to faster approval times and speedier access to new products for patients. The Covid-19 pandemic has only highlighted the importance of global harmonization. Sponsors should embrace these changes, and review their process as a whole, however, and not completely rely on the vendors to provide the total solution for them. It is clear that the publishing vendors in particular will need to make significant changes to their tools, but any software solution requires a well-defined business process to take full advantage of it.
With the introduction of eCTD 4.0 and the first implementations of IDMP, it is clear the next few years will be a busy time for change management within the Life Sciences industry. In the long-run, however, the benefits are clear and will be worth the effort.
Keywords: eCTD v4.0, eCTD 4.0, electronic common technical document 4.0 format, ICH, FDA, regulatory submissions, IDMP, eCTD version 4.0, eCTD 4.0 implementation, eCTD 4.0 timeline, eCTD 4.0 FDA
Content is last updated on 4 October 2022 Tuesday.