Fully automated
PDF validation software
Automatically validate your PDFs against eCTD criteria
Our 1-minute video explains how we automatically validate your PDFs
Want to see automated PDF validation for compliance in action?
Designed to make your organization more productive
Save time by automating cumbersome technical compliance checks
Produce controlled and consistent results
Reduce the risk of a refusal to file and strengthen technical compliance
Automatically check and fix PDF files coming from external sources, such as CROs & external writers
Reduce the dependency on manual desktop tools and drastically reduce associated licensing costs across the organization
Validate early and provide truly submission-ready content to your publishers. Don’t wait until point of publish to validate your PDFs
Check and fix PDFs, regardless of how and where they were originally created
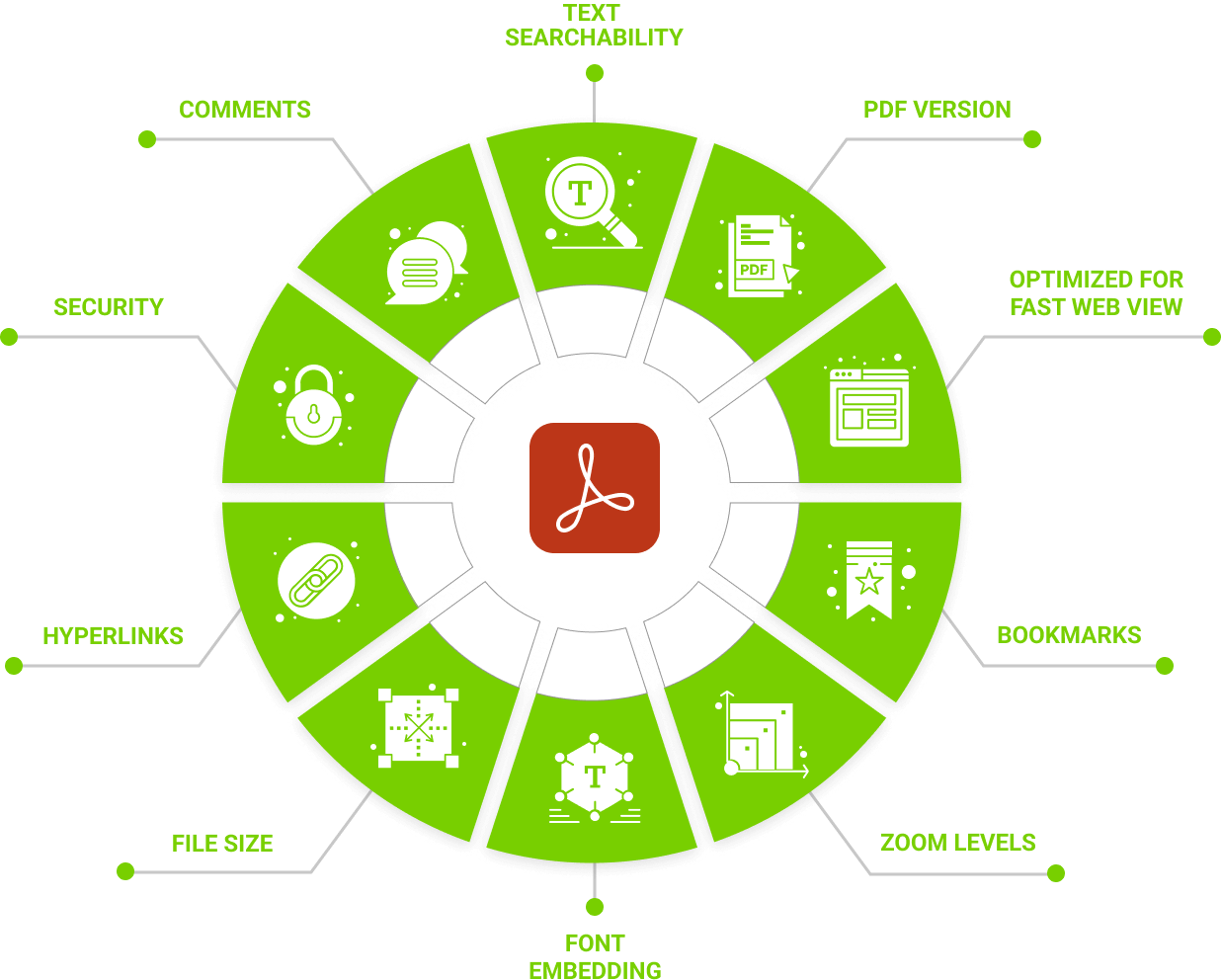
Automatically check & fix
formatting & styling errors in PDF files
- Bookmarks only included to (and open to) the correct level
- Bookmark nesting issues
- Look for broken & no-action bookmarks
- All bookmarks are set to correct zoom level (eg. inherit zoom)
- Check bookmarks exist where necessary
- Ensure bookmarks exist for each ToC entry
- Check first bookmark points to first page
- Check bookmarks point to correct page
- All hyperlinks are the correct colour (e.g. blue)
- No invisible or broken hyperlinks exist
- Relative paths are used for all hyperlinks
- No external links are included
- All hyperlinks are set to correct zoom level (eg. inherit zoom)
- Identify any non-linked blue text
- Check a ToC is included, if needed
- Check hyperlinks point to correct pages
- The PDF file size does not exceed any regulatory limits
- Check no security (passwords or restricted access) is applied
- The PDF version is correct
- The PDF is optimized fo ‘fast web view’
- Check for font and initial view issues
- Check used fonts are embedded correctly
- Check initial page layout and magnification are set correctly
- Page sizes are correct (A4, Letter, etc)
- The initial page orientation is portrait
- There are no blank pages
- Page numbering is sequential
- Ensure headers and first page are not set to draft
- Look for any text remaining in the margins
- The PDF is not empty and contains searchable text
- No track changes exist
- No annotations (of specific types) or comments remain
- No digital signatures are included
The solution?
Automatically validate PDFs and champion submission-ready compliance
Built to handle even the toughest of regulatory compliance standards, DocShifter’s PDFValidator automatically checks, fixes and validates PDF files to remove potential compliance breaches and ensure they’re truly and technically submission-ready.
By reducing the need for human intervention, PDFValidator automates PDF validation reducing workload and accelerating document preparation for your regulatory submissions.
Our PDF Validation Software at a Biotechnology Company
DocShifter helps biotechnology company achieve %60 time saving in document preparation and speed up time to market by %30.
All thanks to automated PDF checking and fixing.
Speak to one of our specialists
Frequently Asked Questions
Which Document Management Systems (DMS) can PDFValidator pull documents to validate content from?
PDFValidator support all major Document Management Systems. For more information, head on over to our Integration section.
Can PDFValidator validate PDFs that are created with other tools (for example Adobe Acrobat, document management systems, ISIToolbox)?
It certainly can. Any PDF file available can be processed by PDFValidator, regardless of how it was created.
Can new checks be added upon request?
Absolutely. DocValidator is updated based on customer feedback and requests. Please let us know if you have any specific requests. If it's not already in our roadmap, we'll be happy to discuss its potential.
Can I receive the list of all the checks that are available?
Yes. Please contact us for more information.
Can I run my PDFs that I receive externally through DocShifter's PDFValidator?
Yes, absolutely. If your PDF documents are created externally (for example by your CRO, or external authors) you can run these through DocShifter's PDFValidator for compliance checks.
You are talking about generating a report after PDF compliance checks. What is this report?
Once PDFs have been analyzed for compliance by PDFValidator, a PDF report can be returned upon choice. This report will show you everything that is analyzed; and will show you where the non-compliance elements were.
You will see 'pass' for everything that is compliant. You can see an example report on the PDFValidator Software Solution page.



