Continuing the LinkedIn Live thought leadership series with Aki Yamaguchi from LORENZ Life Sciences Group.
During this LinkedIn live session, Mr. Yamaguchi and Paul Ireland will discuss the global aspects and the future direction of the electronic common technical document.
What can you expect to see in the video?
- What is/was the main purpose for eCTD?
- What are the global challenges for eCTD, both for the sponsors and the vendors?
- What is the future of eCTD?
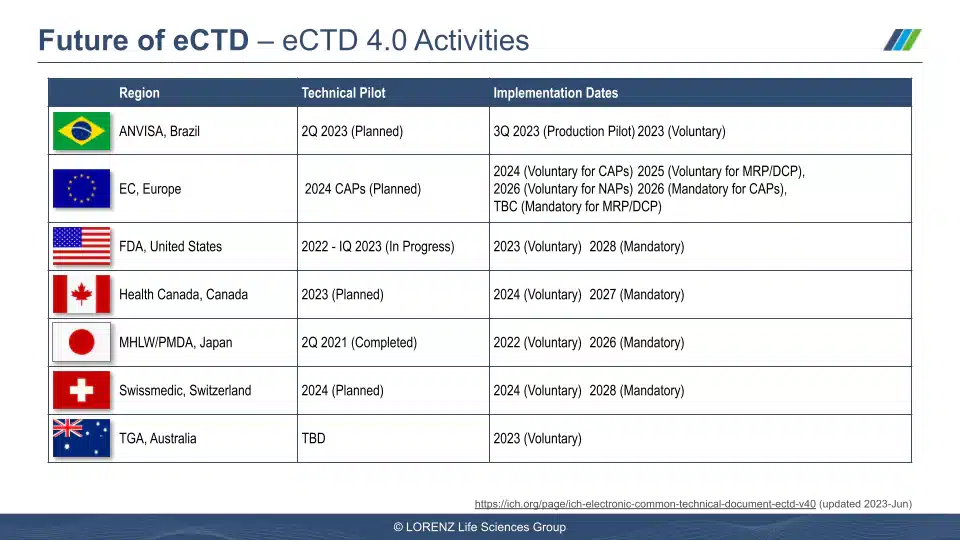
- Are there any learnings from the eCTD 4.0 pilot project at the FDA?
- How about the MHRA and eCTD 4.0?
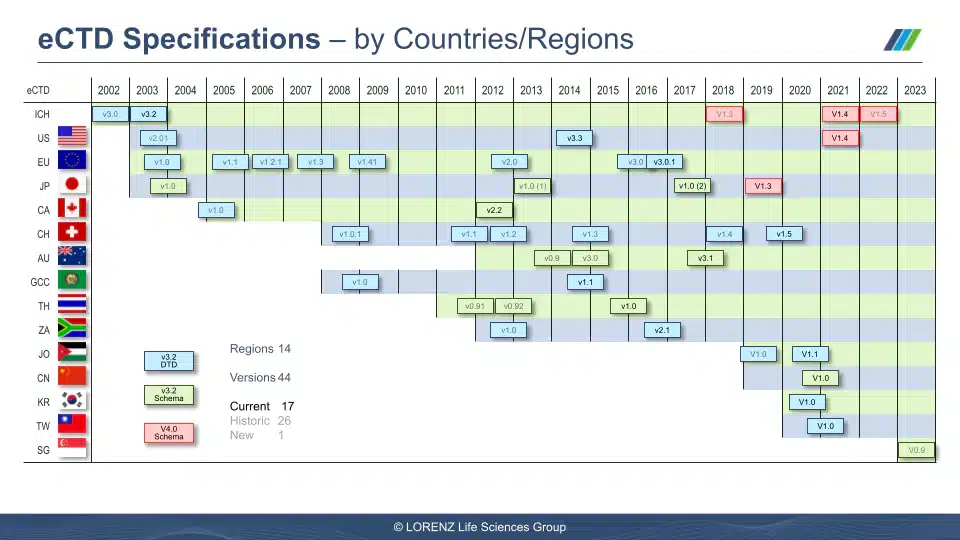
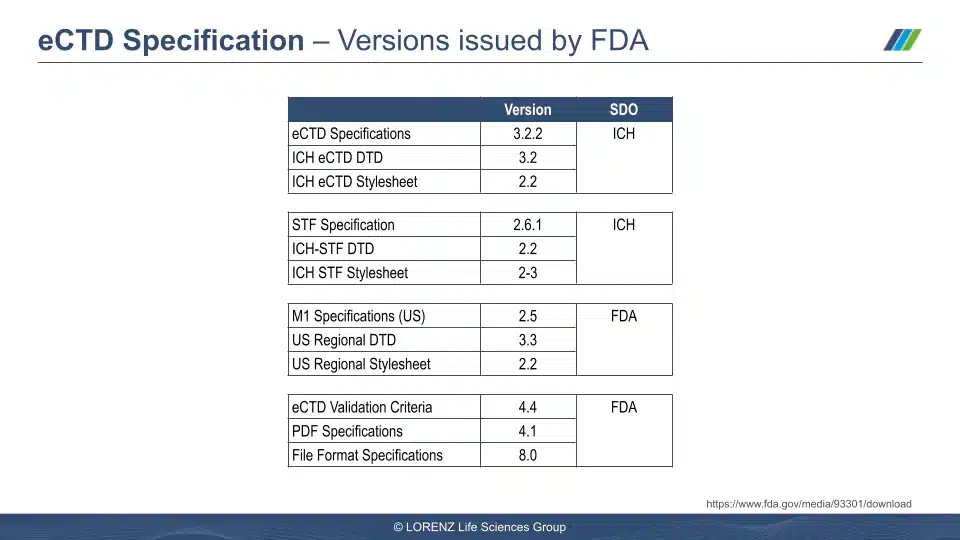
Visuals from the Session
About Aki Yamaguchi
Akira Yamaguchi- Chief Technology Officer at LORENZ Life Sciences Group.
After an international career in management consulting and information technology, Akira joined LORENZ in 1995. Akira’s initial field of activity has been electronic submission projects at regulatory agencies and pharmaceutical companies. In 2003, he became responsible for LORENZ’s overall software product development.
Regarding eCTD, Akira’s involvements are:
- Development of regional eCTD 3.2 specifications (e.g. Australia, Canada, China, Singapore)
- Implementation of agency review systems for eCTD 3.2 (e.g. CDE, Health Canada, US FDA)
- Implementation eCTD 4.0 publishing for industry to participate in pilots for PMDA and FDA.
- Implementation of pilot review system for the FDA eCTD 4.0 pilot