The electronic submission requirements (CDER/CBER) will apply to the following types of submissions:
- ANDAs
- BLAs
- Commercial IND applications
- Master files, such as DMFs.
- NDAs
- All subsequent submissions to these types of applications
For which types of submissions are the requirements optional?
The electronic submission standards are optional but encouraged for the following types of submissions:
- Noncommercial INDs
- Submissions for blood and blood components
- Submissions for promotional materials that are meant for human prescription drugs
What is an NDA?
A New Drug Application (NDA) is the final step by a drug sponsor to the FDA, for the approval required to market a new drug in the U.S
What is an ANDA?
An Abbreviated New Drug Application (ANDA) contains data which is submitted to the FDA for the review and potential approval of a generic drug product in the U.S.
What is a BLA?
A Biologics License Application (BLA), is a Biologics license that is required in the U.S. before a biological product can be introduced into interstate commerce.
What is in a BLA?
- Labeling
- Pre-clinical Studies
- Clinical Studies
- Form FDA 356h
- The product and manufacturing information
- Formulation
- Facility information
- Source material/raw material
- Manufacturing process and controls
- Contamination/cross-contamination information
- Environment assessment or categorical exclusion
What is an IND?
An Investigational New Drug application (IND) is a request for authorization from the FDA to administer an investigational drug or biological product to humans.
What is a DMF?
A Drug Master File (DMF) is a submission to the FDA that provides information from manufacturing to storing of a human drug.
What is a TMF?
A Trial Master File (TMF) is a collection of essential documents for a sponsor to record how they fulfilled their obligations for a clinical trial.
What is an ETMF?
An Electronic Trial Master File (ETMF), is a trial master file in digital format.
What is an ETMF used for?
The ETMF is used for storing and managing documents, images or other digital content for pharmaceutical clinical trials that may be needed for regulatory compliance.
What is an ACTD dossier?
An ACTD dossier is a well-structured Common Technical Dossier for the registration of pharmaceuticals for human use that is submitted to ASEAN regulatory authorities.
What are dates to remember for eCTD?
- Since May 5, 2017, The New Drug Applications (NDAs), Abbreviated NDAs (ANDAs), and Biologics License Applications (BLAs), must be submitted using eCTD format.
- As of May 5, 2018, the Commercial Investigational New Drug Applications (INDs) and Master Files must be submitted using eCTD format.
After these dates, the eCTD requirements for submissions (CDER and CBER) will go into effect and those who do not use eCTD will not be filed or received.
Are you ready for these dates? If not, you better look into a possible solution that will make your eCTD filing much smoother.
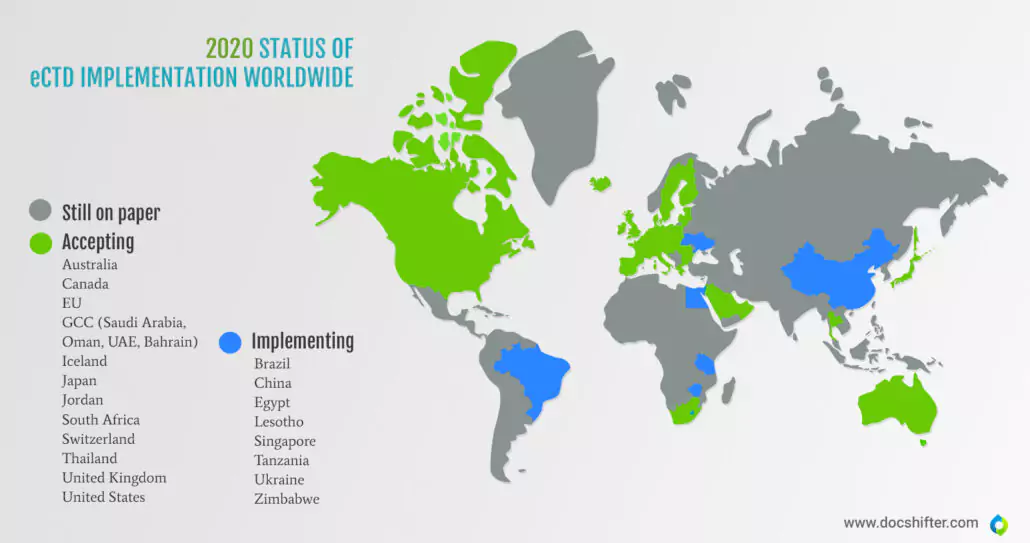
Did you know this? Many countries still submit on paper.
Did you enjoy this blog post? We have more free knowledge-articles available for you.
Thank you very much for reading our blog post, we hope you enjoyed it.
For more free articles on regulatory topics (eCTD, regulatory submissions, RIM, eCTD v4.0, etc.) we invite you to join our free LinkedIn Regulatory Community.
This community is where we share tips & tricks, updates and learnings on regulatory topics. The community currently has 2500 members and is growing quickly.
-
The PDF format specifications checklist for FDA submissions.
- eCTD 4.0: Key Changes & Impact on Submission Content Preparation
If you think this is interesting, I would like to personally invite you to join the Regulatory Professionals Community on LinkedIn. And don’t worry, it is completely free.
About DocShifter
Speed, quality, scalability, and configurability are reasons why Life Sciences organizations choose DocShifter to generate technically compliant, submission-ready PDF. High volume, high-quality document conversion, on-premises, or in the cloud. Super easy to set up. Automate. Centralize. Eliminate manual intervention. Reduce risk. Reduce IT infrastructure costs.
Bonus: We created an FDA PDF format specifications checklist for you, so that you can identify content-related issues as soon as possible to reduce the risk of RTF.
You can download the checklist here.